Oral Absorption
Following extravascular drug administration, the drug needs to be absorbed into the systemic circulation.
Following absorption from the gastrointestinal (GI) tract, the hepatic portal vein passes the drug through the liver, where it undergoes first pass metabolism. For some drugs a large portion of the dose is metabolized by first pass metabolism before the drug gets the chance to reach the systemic circulation.
Following an oral dose, not all the dose administered necessarily reaches the systemic circulation. The fraction of drug which reaches the systemic circulation is termed it’s oral bioavailability.
Therefore, in order to determine the absolute bioavailability, we compare systemic drug exposure, based on determining the area under a concentration vs time profile from the extravascular route to intravenous.
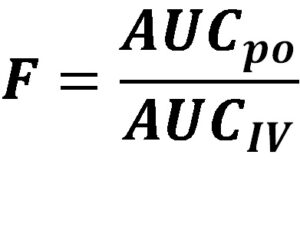
F = bioavailability; AUCpo = area under the concentration-time profile following oral dosing; AUCIV = area under the concentration-time profile following intravenous dosing
Many factors affect drug absorption, some of those may be related to the physicochemical properties of the drug itself. How soluble is the drug and will solubility across the different pH range of the GI tract limit available drug for absorption? What part of the GI tract is the drug absorbed? For many drugs, the small intestine is the primary site of absorption due to it’s high surface area and active transporters. Is the drug permeable through membranes and able to be taken up readily? Other factors in a clinical setting could include food or drug interactions. Food can delay gastric emptying, stimulate bile flow, change gastrointestinal (GI) pH, increase splanchnic blood flow, change luminal metabolism of a drug substance, and physically or chemically interact with a dosage form or a drug substance. Does the formulation limit absorption? Are there any concomitant medications could interact with enzymes and transporters or alter pH of the gastric fluid?
There are many questions that need to be answered in order to understand the absorption process in order to assess how various factors may influence PK exposures, safety and efficacy of a particular dosing regimen in clinical drug development and intended patient populations.
If you have a drug development program that you would like to discuss with us, please get in touch.